Central Forces: Spring-2025
HW 10 : Due W5 D5
- Eigenvalues for Different Systems
S0 5254S
Fill in the following table with the appropriate eigenvalues for each operator for each system.
\[ \begin{matrix} ~\\L_z\\~\\L^2\\~\\H \end{matrix} \begin{vmatrix}\left|{m}\right\rangle &\left|{\ell,m}\right\rangle &\left|{n,\ell,m}\right\rangle \\ \underline{\text{particle on a ring}}& \underline{\text{particle on a sphere} }& \underline{\text{Hydrogen atom}}\\~\\ \\~\\ \\~\\~\\ \end{vmatrix}\]
Write the Hamiltonian for each of the following systems explicitly in the position representation (i.e., differential operators).
\[ H\begin{vmatrix}\left|{m}\right\rangle &~&~&\left|{\ell,m}\right\rangle &~&~&\left|{n,\ell,m}\right\rangle \\ \underline{\text{particle on a ring}}&~&~& \underline{\text{particle on a sphere} }&~&~& \underline{\text{Hydrogen atom}}\\\\ \\ \\ \end{vmatrix} \]
- SP not Hybrid
S0 5254S
A hydrogen atom is initially in the state \(\left|{\Psi(t=0)}\right\rangle =\frac{1}{\sqrt{2}}\left(\vert 1,0,0\rangle +\vert 2,1,0\rangle\right)\).
- If you measure the energy of this state, what possible values could you obtain?
- What is \(\left|{\Psi(t)}\right\rangle \), where \(t>0\)?
- Calculate the expectation value \(\langle\hat L^2\rangle\) in this state, as a function of time. Did you expect this answer? Please explain your reasoning.
Write \(\left|{\Psi(t)}\right\rangle \) in wave function notation.
- Hydrogen, Version 1
S0 5254S
A hydrogen atom is initially in the superposition state \begin{equation} \vert \psi(t=0) \rangle = \frac{1}{\sqrt{14}}\vert 2,1,1\rangle - \frac{2}{\sqrt{14}}\vert 3,2,-1\rangle + \frac{3}{\sqrt{14}}\vert 4,2,2\rangle . \end{equation}
- What are the possible results of a measurement of the energy and with what probabilities would they occur? Plot a histogram of the measurement results. Calculate the expectation value of the energy.
- What are the possible results of a measurement of the angular momentum operator \(L^2\) and with what probabilities would they occur? Plot a histogram of the measurement results. Calculate the expectation value of \(L^2\).
- What are the possible results of a measurement of the angular momentum component operator \(L_z\) and with what probabilities would they occur? Plot a histogram of the measurement results. Calculate the expectation value of \(L_z\).
- How do the answers to (a), (b), and (c) depend upon time?
- Confidence Rating
S0 5254S
After solving each problem on the assignment, indicate your answers to the following questions for each problem. Answer for the problem as a whole, even if the problem has multiple parts.
- Question Confidence How confident are you that you are interpreting the problem the way the instructor intends?

For the rest of the questions, assume you have interpreted the problem correctly - Problem Confidence How confident are you that you could independently come up with a correct solution process to a similar problem on a future problem set?

- Answer Confidence How confident are you that your final answer to this question is correct (not solution process)?

- Makes Sense To what degree do you understand how your answer fits (or does not fit) the physical or mathematical situation of the problem?
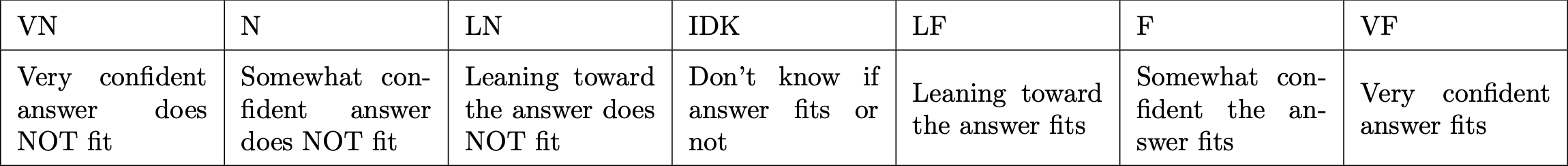
- Question Confidence How confident are you that you are interpreting the problem the way the instructor intends?